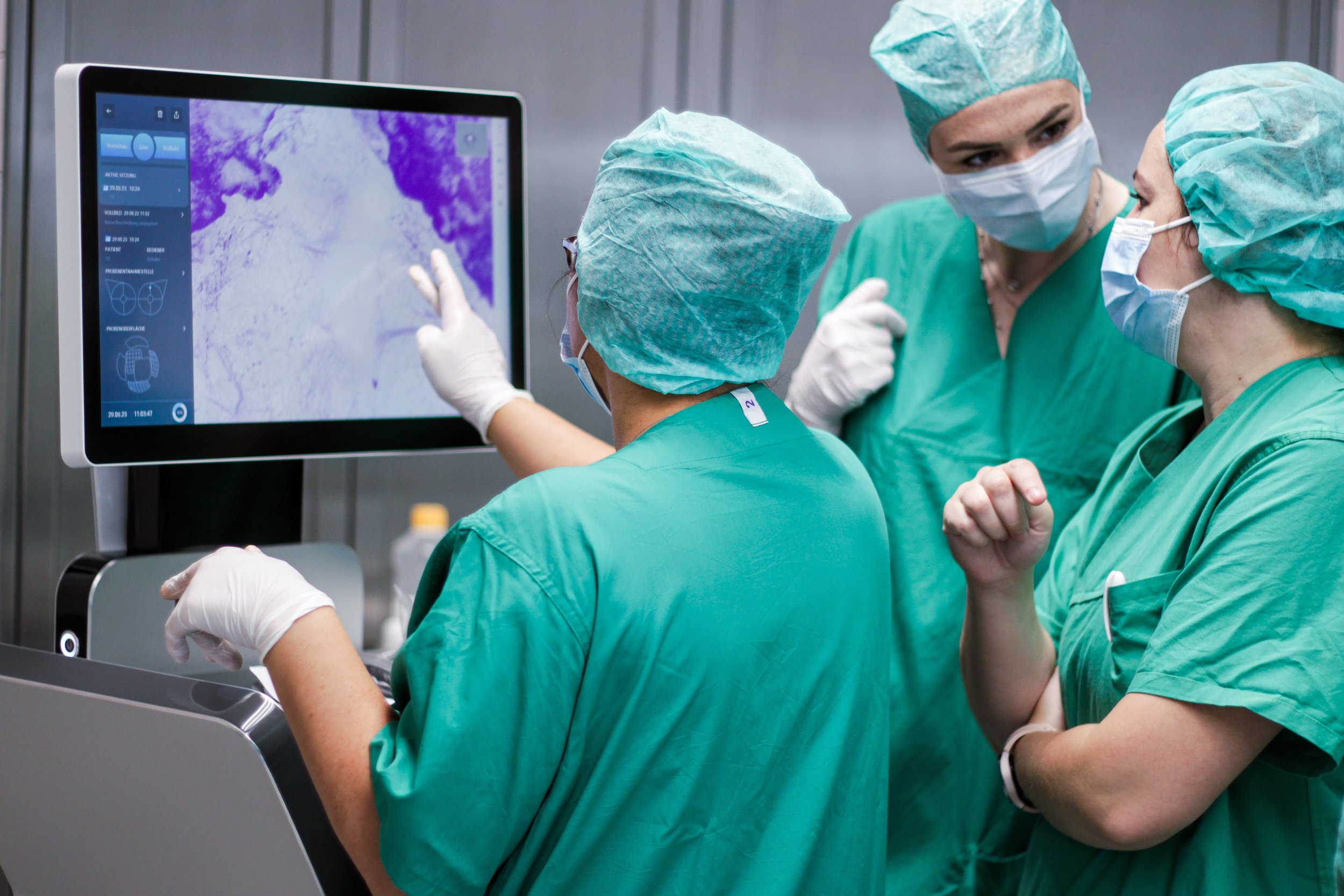
Working at SamanTree
An impactful work experience with innovative technology and a strong patient focus
Open positions
-
Roles and Responsibilities
As an IT Integration Engineer, you will interact with our customers’ IT teams to present the Histolog solution and its deployment process. In partnership with our customers, you will establish and execute deployment and integration plans customized to each customer. You will gather feedback on customers’ IT integration requirements and communicate with our R&D department to support continuous improvement of our products and solutions. You will ensure the ongoing performance and reliability of the company’s software solutions by planning and performing the necessary maintenance at customer locations.
As a secondary responsibility, you will execute some of the company’s IT administration tasks.
As SamanTree Medical is a start-up/scale-up company, flexibility is essential for candidates in this position. The ultimate goal is to gradually focus the geographic coverage area as the company grows.
Responsibilities:
Responsible for integration of SamanTree Medical's hardware and software products within a medical lT environment of client hospitals.
Responsible for the first level of technical support of SamanTree Medical's solutions running on client sites.
Responsible for configuration and maintenance of SamanTree Medical's network infrastructure and cloud services, as well as execution of the company’s IT administration tasks.
SKILLS, EXPERIENCE AND QUALIFICATIONS
Education
B.Sc./Master in Science, Engineering, Engineering management, or related discipline
Experience
At least 3 years in lT infrastructure and system administration function, or an equivalent experience. Experience of work with hospitals IT infrastructure will be a valuable asset.
Technical Skills
Mastery of both Windows and Linux (and other UNIX) operating systems administration
Good knowledge of Microsoft 365, Intune, Windows Defender, SharePoint, Powershell/Bash scripting
Experience with container management (Docker, Kubernetes…) and with virtualization solutions (VMWare, Citrix, …)
Proficiency with shared storage technologies
Knowledge of Network routing and protocol and secure communication protocols (e.g. SSH, SFTB SSL/TLS)
Other skills & requirements
Handles detailed, complex concepts and problems, balances multiple tasks simultaneously, and makes rapid decisions regarding administrative issues.
Establishes professional relationships with Management, employees, suppliers, partners and customers.
Plans and meets deadlines.
Fluent in French & English
Travel up to 30% domestic & international
Our offer
A full time job
Attractive and competitive salary package in line with your profile and experience
A dynamic and collaborative work environment
Opportunities for professional growth and development
A role that directly contributes to the improvement of global healthcare outcomes
Equal Opportunity Statement
We are all proud to work for such a great company as Samantree Medical. And we would be even prouder to have you on board. Whoever you are, wherever you come from, regardless of your race, religion, color, sex (including pregnancy and gender identity), national origin, political affiliation, sexual orientation, marital status, disability, genetic information, age, membership in an employee organization, retaliation, parental status, military service, or other non-merit factor. Please apply if you see a position that makes your heartbeat, and if you feel the role is appealing to you. If you’re smart and good at what you do, come as you are.
SamanTree is an emerging company with significant potential in the MedTech sector. For individuals seeking challenges, SamanTree offers opportunities for growth both nationally and internationally. We value inquisitive and highly committed individuals who are dedicated to collaborating towards making SamanTree a success.
Join a team that’s setting new standards for healthcare professionals and their patients worldwide. Together, let’s transform the future of Intra Operative Margin Assessment with the Histolog Scanner.
Interested ? Please send your application to hr@samantree.com -
The Software Quality Assurance (Test) Engineer is responsible for design, implementation, execution and maintenance of processes of the technical quality assurance of SamanTree Medical software products or product components and plays an important role in the products development.
Responsibilities:
Assures efficient testing and continuous integration within SamanTree Medical software code base throughout different software projects of the company. Maintains existing automated tests and extends them for the new test cases.
Works in close collaboration with the software development team to ensure consistency of the company’s software with established requirements and to provide feedback to developers and engineers.
Develops test procedures (both manual and automated) and ensures proper execution of both routine tests for running development tasks and release tests for software products and components.
Specifies software use cases and test protocols, executes tests, maintains test reports and technical product documentation in compliance with ISO 13485, IEC 62304 and applicable regulatory requirements.
Performs issue reporting in the company’s defect management system (Atlassian Jira). Ensures testing to support efficient management and resolution of software defects.
SKILLS, EXPERIENCE AND QUALIFICATIONS
Education
B.Sc./Master in Science, Engineering, Engineering management, or related discipline
Experience
2+ years in relevant positions, ideally in the medical device industry
Technical Skills
Knowledge of the software development processes, experience with agile practices.
Experience with development and maintenance of automated tests for continuous integration.
Mastery of both Windows and Apple Mac operating systems. Experience with Linux (and other UNIX) is a plus.
Programming languages: proficiency with Java and Java frameworks, understanding of C, C++.
Experience with User Interface testing (manual and automated). Experience with JavaFX, CSS, Scene Builder.
Good comprehension of performance testing and of the monitoring tools.
Solid experience with:
relevant frameworks and automation tools (TestFX, Cypress, JUnit, etc)
revision maintenance system (Git)
issue management system (Atlassian Jira)
integrated development environment (IntelliJ Idea, Eclipse, etc.)
build management and continuous integration (JFrog Artifactory, JetBrains TeamCity, GitLab CI, Jenkins)
software specifications, modeling and test management tool (Sparx Enterprise Architect , TestRail)
performance profiling
Containerization and orchestration tools (such as Docker, Kubernetes) are a plus.
Languages
English (spoken and written).
French (spoken).
Russian (spoken) is a plus.
Soft Skills & personality
Autonomous and proactive, with a strong sense of initiative.
Self-organization, motivation, and commitment to continuously improving the company’s products and services
Motivation and ability to contribute to a collaborative, results-driven environment.
Experience in a start-up or other small organizations is a plus.
Handles detailed, complex concepts and problems, and balances multiple tasks simultaneously.
Establishes professional relationships with Management, employees, suppliers, and customers.
Plans and meets deadlines. Maintains a flexible work schedule.
Our offer
A full time job
Attractive and competitive salary package in line with your profile and experience
A dynamic and collaborative work environment
Opportunities for professional growth and development
A role that directly contributes to the improvement of global healthcare outcomes
Equal Opportunity Statement
We are all proud to work for such a great company as Samantree Medical. And we would be even prouder to have you on board. Whoever you are, wherever you come from, regardless of your race, religion, color, sex (including pregnancy and gender identity), national origin, political affiliation, sexual orientation, marital status, disability, genetic information, age, membership in an employee organization, retaliation, parental status, military service, or other non-merit factor. Please apply if you see a position that makes your heartbeat, and if you feel the role is appealing to you. If you’re smart and good at what you do, come as you are.
SamanTree is an emerging company with significant potential in the MedTech sector. For individuals seeking challenges, SamanTree offers opportunities for growth both nationally and internationally. We value inquisitive and highly committed individuals who are dedicated to collaborating towards making SamanTree a success.
Join a team that’s setting new standards for healthcare professionals and their patients worldwide. Together, let’s transform the future of Intra Operative Margin Assessment with the Histolog Scanner.
Interested ? Please send your application to hr@samantree.com
-
RESPONSABILITIES
Reporting to the Software Architect, you will be responsible for designing and implementing the Company’s medical software solutions. You will be involved in the entire software development lifecycle, from requirement analysis to deployment.
Your main responsibilities are:
Collaborate daily in the product development, from the idea to the customer support.
Design and develop the company’s software solutions with the development team.
Select appropriate tools and technologies to meet functional and technical requirements.
Implement Front-End (JavaScript, TypeScript, VueJS) and Back-End features using Java, Spring, PostgreSQL.
Ensure code quality through code reviews, automated testing, and CI/CD pipelines.
Maintain and improve technical documentation, ensuring compliance with ISO 13485, IEC 62304, FDA, and CE regulations.
Diagnose and fix software defects.
Contribute to project planning and workload estimation.
Participate in technical decisions and software architecture improvements.
PROFILE
You have a Bachelor's or Master’s degree in Computer Science (or equivalent) and at least 5 years of experience in web development.
You are proactive and collaborate effectively with developers, managers, and stakeholders.
You master Java and Spring for Back-End development and TypeScript with a modern framework (VueJS, React, or Angular) for the Front-End.
You are comfortable with relational databases (PostgreSQL, SQL).
You work with modern tools: Docker, Git, Gitlab CI/CD, testing frameworks.
You have a working knowledge of Linux
You enjoy solving complex problems and working in a dynamic environment.
You fluently communicate in English. French is a plus.
You are ready to travel occasionally.
OFFER
A challenging and diversified position within a high-potential fast-growing innovative medical device company.
The opportunity to participate in the development of the company.
To work in a human size, dynamic, respectful, and professional environment and cont
A role that directly contributes to the improvement of global healthcare outcomes.
An attractive compensation package in line with the position responsibilities and your experience
Interested ? Please send your application to hr@samantree.com
-
Reporting to the VP RA/QA, he/she will implement and maintains ISO 13485 quality management systems, create and maintain all quality processes/documentation, ensure compliance of personnel to the QMS through coaching and training, fulfill customers' requirements on quality issues and work closely with the management team of SamanTree Medical in fulfilling all of the above.
The position is office based (Awans, Belgium) involving EU travel to different SamanTree entities and to suppliers.
Responsibilities
Maintain and trends Quality Objectives,
Oversee and maintain compliance with ISO 13485:2016 and – when applicable – other Quality Management Systems,
Acts as management representative as defined in ISO 13485:2016,
Promotes Quality Systems, Quality Objectives and Quality Policy,
Owner of the internal and external (supplier) audit process,
Host 3rd party audit (notified body, competent authority and customer),
Owner of the complaint and feedback process,
Work in collaboration with the operations departments to prepare manufacturing and quality agreements with external providers and suppliers,
Identity area’s of improvements and implement the improvements,
Identifies risks and performs trending of complaints, deviations, and audit findings, and identifies appropriate corrective and preventive measures,
Effectively interact with all departments of Samantree Medical SA Medical to resolve quality issues in a timely manner,
Leads the periodic Management Review meetings, and
Other duties as assigned by direct supervisor.
Skills, Qualifications and Experience
Bachelor's degree in a technical related field
At least 10 years of work experience in an ISO 13485 regulated industry.
Excellent knowledge in ISO 13485:2016
Certified ISO 13485 auditor
Good understanding of medical devices regulatory environment (MDR and IVDR)
Excellent communication, organizational and problem-solving skills
Ability to plan, organize and execute activities in a relevant field
Hands-on, self-driven and autonomous.
Team oriented, adaptive and customer oriented.
Strong attention to detail and respect of regulatory obligations.
Fluent communication in English (Written / Oral). French, in particular, and any other language is a plus.
Willing to travel up to 20%, in possession of a driving license and in possession of a valid EU work permit.
Our offer
A full-time job
Attractive and competitive salary package in line with your profile and experience
A challenging and diversified position within a high-potential fast-growing innovative medical device company.
Product training provided in Switzerland.
A human-size, dynamic, respectful, and collaborative work environment
International exposure, with learning and development opportunities.
A role that directly contributes to the improvement of global healthcare outcomes
How to Apply
Please submit your resume and a cover letter detailing your relevant experience and language skills to hr@samantree.com
Applications will be reviewed on a rolling basis.
Equal Opportunity Statement
We are all proud to work for such a great company as Samantree Medical. And we would be even prouder to have you on board. Whoever you are, wherever you come from, regardless of your race, religion, color, sex (including pregnancy and gender identity), national origin, political affiliation, sexual orientation, marital status, disability, genetic information, age, membership in an employee organization, retaliation, parental
status, military service, or other non-merit factor. Please apply if you see a position that makes your heartbeat, and if you feel the role is appealing to you. If you’re smart and good at what you do, come as you are.
SamanTree is an emerging company with significant potential in the MedTech sector. For individuals seeking challenges, SamanTree offers opportunities for growth both nationally and internationally. We value inquisitive and highly committed individuals who are dedicated to collaborating towards making SamanTree a success.
Join a team that’s setting new standards for healthcare professionals and their patients worldwide. Together, let’s transform the future of Intra Operative Margin Assessment with the Histolog Scanner.
No open role for you right now ?
We’ll be happy to consider your application for further role openings. Please feel free to send us your resume and motivations via e-mail: